FDA Approves Dexcom G6
Editor’s Note: This is a breaking news story and will continue to be updated as more information becomes available. Read Beyond Type 1’s interview with Dexcom CEO Kevin Sayer here.
The U.S. Food and Drug Administration today approved a version of Dexcom’s revolutionary G6 integrated continuous glucose monitor (CGM) system that does not require fingerstick calibration and is designed to be integrated with future insulin pump systems. Its targeted ship date is June 4, 2018.
The G6 is the first integrated CGM system to be approved by the FDA and may streamline the approval process for other similar integrated CGM systems. The device itself represents another leap forward in diabetes technology for Dexcom, whose G5 system was approved in 2016.
New Dexcom G6 Features
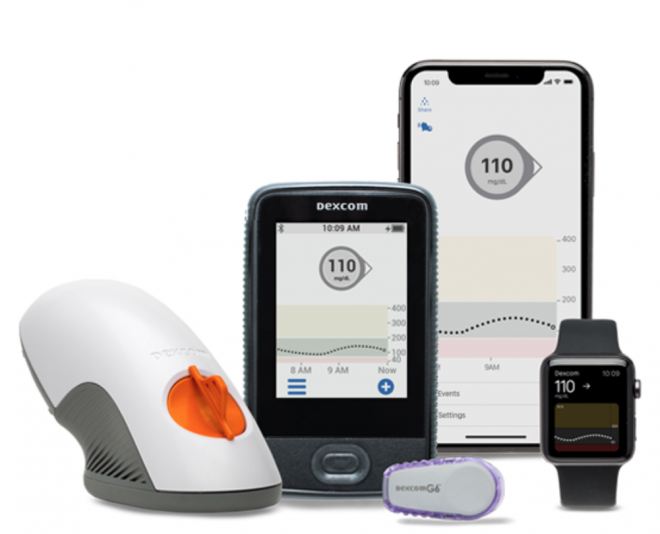
- Fingerstick Elimination—No fingersticks are needed for calibration or diabetes treatment decisions.
- Easy Sensor Applicator—Complete redesign of the sensor applicator allows for one-touch, simple insertion.
- Discreet and Low Profile—A redesigned transmitter with a 28 percent lower profile than previous generation Dexcom CGMs makes the device comfortable and easy to wear under clothing.
- Acetaminophen Blocking—New feature allows for more accurate blood glucose readings with no medication interference.
- Predictive Low Alert—New alert feature predicts hypoglycemia before it hits to help avoid dangerous low blood sugar events.
- Extended 10-Day Sensor—10-day sensor allows for 43 percent longer wear than previous generation Dexcom CGMs.
Watch Beyond Type 1’s unboxing video of the Dexcom G6 Demo Kit on Facebook Live.
“As a factory-calibrated, real-time CGM system with exceptional accuracy, the Dexcom G6® will be transformative for people with diabetes, who will no longer be required to prick their fingers for diabetes management,” said a member of Beyond Type 1’s Scientific Advisory Council Daniel DeSalvo, MD, pediatric endocrinologist. “I can tell you as someone who has type 1 diabetes myself, with all of its features and benefits, the Dexcom G6® is the CGM device I have been anticipating for the last twenty years. This CGM system will help to alleviate the burden of diabetes management while improving the lives of people with diabetes.”
The Dexcom G6 is a patch device and about 30 percent thinner than its predecessor. Around the size of a quarter, it is applied to the skin of the abdomen. A small sensor continuously measures blood glucose levels and transmits real-time readings every five minutes to a compatible display device, such as a cell phone app or Dexcom’s touchscreen receiver. When integrated with an automated dosing system, a rise in blood sugar triggers the release of insulin from the pump.
Unlike other CGM systems, the G6 comes factory calibrated, eliminating the need for users to set baseline levels with a fingerstick test. In addition, the updated sensor probe minimized interference with acetaminophen (Tylenol). Diabetes educators and treatment professionals have long known that acetaminophen can lead to elevated CGM readings. Despite product warnings to that effect in the past, many patients have remained unaware still of the interaction and faced confused blood sugar readings form their CGM systems.
The G6 is approved for children ages two and older and adults with diabetes. The FDA evaluated data from two clinical studies that included 324 patients. Both studies included multiple clinical visits within a 10-day period. During the visits, the G6 readings were compared to laboratory test measures for blood glucose levels.
According to the FDA, the authorization also classifies integrated CGM systems in such a manner that should allow developers of future systems to bring their products to the market in the least burdensome manner possible, streamlining the approval process.
Dexcom has indicated that it will begin shipping to patients soon—contact your Dexcom representative for specifics. Beyond Type 1 will continue with additional coverage as more information becomes available.
FAQ
You asked, then we asked Dexcom! Answers to popular community questions about the G6 rollout.
Q: What is the expected timeline for G6 availability and how much will it cost?
A: We plan to commercialize G6 later this year and will provide details on its availability as we prepare and approach the launch date.
Q: Will there be differences in cost and coverage from the G5?
A: We are working to finalize cost and coverage, more details to come as we approach launch.
Q: What will the transmitter battery life be?
A: The G6 transmitter life will be consistent with that with G5 (about three months).
Q: Can you restart the sensor once inserted?
A: The sensor can be used for its labeled 10-day life. Based on the special controls the FDA established, devices in the category G6 has to be classified must include appropriate measures to ensure that disposable sensors cannot be used beyond their claimed sensor wear period.
Q: Can you calibrate if desired?
A: G6 offers the ability to accept calibrations, which we expect will happen in rare circumstances. This feature also allows our insulin delivery partners to include calibrations in their system if they so choose.
Q: Is there a timeline for approval in Canada? Any other international approvals pending?
A: The Dexcom team has developed a detailed rollout plan to a number of OUS geographies, including Canada. We will elaborate on these plans as we obtain the appropriate international regulatory clearances for G6.
Q: Will G6 integrate with the t:slim x2 insulin pump from Tandem?
A: As with any next generation system, we will look to integrate G6 with all of our partners as soon as possible. As you can see from the FDA’s approval, G6’s interoperability with other devices was a priority in this decision. Specific to t:slim X2, we will work hard with Tandem to commercialize a G6 compatible system, and believe that because that insulin pump has the ability to be upgraded in the field, we will be able to bring that feature to t:slim pump users soon after it obtains the appropriate regulatory approvals.
Other Dexcom product updates
Disposable CGMs in the works
 Dexcom has partnered with Verily to produce a fully disposable, smaller CGM products. The first generation will launch after the Dexcom G6 sensor. The sensor is slightly wider although flatter than the previous version. The smaller second generation version is expected to be cheaper and both are expected to send data to a smart phone and not require calibration via fingerstick.
Dexcom has partnered with Verily to produce a fully disposable, smaller CGM products. The first generation will launch after the Dexcom G6 sensor. The sensor is slightly wider although flatter than the previous version. The smaller second generation version is expected to be cheaper and both are expected to send data to a smart phone and not require calibration via fingerstick.
Dexcom G5 Medicare coverage
There is currently no update on when Medicare users will be able to use Dexcom Share since Medicare will not pay for a CGM if the phone app is used for remote monitoring.
Read the interview with Dexcom CEO Kevin Sayer and learn more about what this technology means.



 Donate today to Beyond Type 1!
Donate today to Beyond Type 1!


