Insulin Pump Rundown
WRITTEN BY: BT1 Editorial Team
You’re ready to choose an insulin pump, but which one? Should you go with a more traditional pump where you can monitor and control all of your insulin delivery settings directly? Are you looking for a model that works with a blood glucose monitor (BGM) and allows you to administer insulin via a remote? Maybe you need a pump that offers integration with a continuous glucose monitoring (CGM) system. What about size? Color? To use tubes or not to use tubes?
Depending on what country you live in and what your healthcare and/or insurance plan covers, you may be lucky enough to have several options available to you, so we’re here to help you figure out what may be best for you. Here’s a look at what’s available and the various features of each.
Newest Pumps at a Glance
| Insulet’s Omnipod 5 | Medtronic’s MiniMed 770G | Tandem’s t:slim X2 | Roche’s Accu-Chek Combo | |
 |
  |
   |
|
|
| Notable Features |
|
|
|
|
Click on any of the pump manufacturers below to learn more about past, current and future offerings
Insulet Pumps►
Current Generation



Omnipod 5
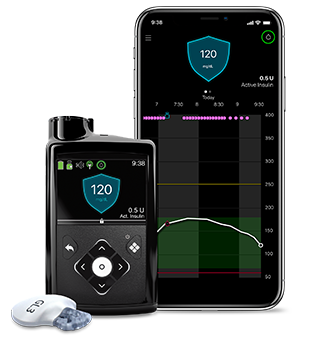
The Omnipod 5 is the first and only tubeless insulin pump technology with a closed-loop “automated insulin delivery” system (AIDs). While it includes many of the same features as the Omnipod DASH system, it integrates with the Dexcom G6 continuous glucose monitor technology to automatically adjust insulin dosing based on real-time blood glucose data from your connected Dexcom CGM system. First approved by the FDA on January 28, 2022, it’s full-market release was announced on August 2, 2022.
Omnipod DASH
This system was cleared by the FDA in June 2018. The DASH PDM received an upgrade from the UST4000 in the form of an Android phone to include a touchscreen interface and Bluetooth technology resulting in a follow feature similar to Dexcom’s Share technology. According to Insulet, this tubeless pump received an IP28 rating for up to 25 feet for an hour at a time. (Keep in mind that the PDM is not waterproof!) English-speaking users have access to a Calorie King database for logging carb counts, and DASH users can access other app integrations like Onmipod DISPLAY (which allows for pump control from a smartphone) and Omnipod VIEW (which allows users to share their pump information with followers). If you are looking for a pump system that resembles a smartphone, this setup might work for you—although it means carrying a PDM that is another piece of technology taking up space in your purse or your pocket.
In Beyond Type 1’s announcement about the Omnipod DASH, Tessa Mellinger, a college student with type 1 diabetes who was part of the DASH user testing and development, said, “The PDM display is easy to read, the steps to managing basal rates are user friendly, and the food library is really helpful. The fact that it looks like a smartphone makes it cool to carry and may help eliminate those awkward questions from strangers.”
Previous Model


Insulet Omnipod UST400
Omnipods are tubeless pumps that stick to the skin via an adhesive patch. This pod contains a small, automated cannula and is filled with up to 200 units of insulin immediately prior to insertion of the pod. A hand-held personal diabetes manager (PDM) acts as a allows for control of injections, displays your levels and data, allows you to set warnings. In the Omnipod UST4000, the PDM has a built-in Freestyle Blood Glucose Meter (BGM) that eliminates the need to carry two separate pump/meter devices, but it doesn’t come with CGM integration.
The Omnipod is a great option for those users who are put off by tubing. Each pod lasts for 72 hours and must be replaced when that alarm goes off, a consideration for people with diabetes (PWDs) who use less than 200 units of insulin in a three-day period if the recommended protocol for insulin withdrawal is not followed exactly when disposing of the pod. The tubeless setup can have a greater margin of error for kinking, which can result in having to dispose of the pod early, wasting insulin and/or having to go through several pods before the angle of insertion is achieved for insulin delivery. The Omnipod is waterproof and can be worn while swimming or bathing without disrupting insulin delivery.
Next Generation
Omnipod Horizon Automated Glucose Control System
Since 2017, Insulet has been in the development phase of its hybrid closed-loop technology. Incorporating a Dexcom CGM into the Onmipod system and applying a predictive algorithm resulted in a safe APS platform for study participants with type 1, as Beyond Type 1 reported in 2018. Both adults and children had blood glucose levels in range more than 70 percent of time. As of December 2019, Insulet announced the start of the pivotal trial for Omnipod Horizon, with an expected launch in the second half of 2020.
Medtronic Pumps►


Current Generation
Medtronic MiniMed™ 770G – The 770G is Medtronic’s second-generation hybrid closed-loop pump, having been the first to market with the MiniMed 670G. The Smartguard Auto Mode system automatically adjusts background insulin amounts every 5 minutes to prevent highs and lows through its integration with Medtronic’s Guardian CGM sensor. The MiniMed Mobile App allows users to see insulin delivery and blood sugar level trends, as well as high and low glucose alerts, via mobile phone. The CareLink Connect App allows users to share blood glucose (BG) levels with friends and family, plus pump and CGM data can be automatically shared with the user’s healthcare provider if desired. The 770G is approved for ages 2 and up.
Next Generation
Medtronic 780G – This software upgrade, currently under FDA review, doesn’t yet have a launch date. Those who start on the 770G system before it is available will receive a no-cost remote software upgrade when approved.
Previous Models
Medtronic MiniMed™ 670G – The 670G is one of Medtronic’s pumps reaching for “artificial pancreas” status, and this system was the first hybrid closed-loop system on the market. Users still have to bolus, but the pump predicts basal rates. The auto-mode option automatically adjusts basal insulin delivery every five minutes, based on information about BG levels it receives from the Guardian CGM. Using Medtronic’s SmartGuard technology, this system catches lows by stopping your insulin delivery up to 30 minutes before you reach your pre-selected low limit, then it will automatically deliver insulin again after recovery. The 670G is approved for ages 14+ because it has a total daily dose requirement of at least 8 units a day. As of February 26, 2018, The Guardian™ Sensor 3 received FDA approval for use on the upper arm to offer more flexibility and accuracy.
Medtronic 630G – Medtronic boasts a more user-friendly design over the 530G with this model, which also features remote bolusing from the integrated meter (the 530G doesn’t). The 630G is another pump that utilizes SmartGuard technology to take predictive action, reducing the risk of complications from diabetes with notifications and alarms 30 minutes before you trend low or high, and the system will pause insulin delivery up to two hours if you are trending low. Also, the meter automatically sends readings to the pump through a Bluetooth connection. This system includes a new pump designed with many consumer-oriented features such as customizable alerts and alarms, additional waterproofing, and the ability to remotely bolus from the meter. Pump skins and accessories are available should you want to jazz up your pump!
Medtronic 640G – This European/Australian pump system from Medtronic offers a few new features over Medtronic’s 530G system. It works with a Guardian 2 link transmitter and Enlite sensor that allows continuous monitoring of your blood glucose levels and is integrated with a Contour Next LINK BCM from Bayer that communicates wirelessly with the MiniMed unit and offers remote bolusing. Like Medtronic’s other products, this pump also shares data through the Medtronic CareLink software.
The biggest upgrade with this model is the introduction of SmartGuard technology, which will suspend insulin delivery if hypoglycemia is predicted by CGM to occur within 30 minutes. The system automatically resumes insulin delivery once blood glucose levels start to recover. The results of the SMILE study, presented at ATTD in February 2019, indicated the effectiveness of the SmartGuard Suspend before low technology in reducing episodes of hypoglycemia in adults with type 1 after six months of use.
Medtronic Minimed Veo – This pump is essentially the non-U.S. version of the MiniMed Revel (being discontinued – see below), offering real-time information with built-in CGM technology. The Revel also has a customizable early warning system that can be set to alert you of oncoming low and high blood sugar. One great benefit of an integrated insulin pump and CGM system is that you only have to deal with one company, making for smoother synchronization and troubleshooting. It offers the same combination of real-time information via a linked BGM and an integrated CGM sensor. It is not waterproof, doesn’t offer food-tracking options, and works with CareLink software.
Medtronic MiniMed Paradigm Revel (discontinued) – Before the advent of the 670G, steady, real-time information was the main selling point of this model. Users were alerted to low and high trends on a pump with a simple LCD-backlit screen. The Revel is no longer being sold as of late 2018.
Medtronic 530G (discontinued) – While alerts are helpful, the Medtronic 530G paused insulin delivery for up to two hours when no responses were recorded (like while users were asleep), helping to avoid nighttime lows. This was handy because nighttime alarms were difficult to hear on the device. While the pump worked great with the online CareLink program (and was integrated with MiniMed Connect, an app that allows for monitoring via cell phone), that the data was not downloadable to other programs like Unicare. Some users reported struggling with the quiet alerts from the CGM, and there is evidence that the Enlite sensor is not as accurate as the Dexcom sensor. Unfortunately, the 530G system only suspends insulin delivery once hypoglycemia was reached.
Despite 93 percent of MiniMed users who say the MiniMed 530G pump let them feel more secure in treating their diabetes, this pump is being discontinued as of the last quarter of 2018.
*Note on older Medtronic Minimed pumps: 512 and 712 – Have you checked out the Beyond Type 1 Guide to DIY Looping? Dana Lewis, one of the creators of the Open APS, described the process for using older Medtronic pumps in a DIY loop using the Open APS during an interview with Beyond Type 1. In the US, Medtronic 512 or 712 pumps can be used with a separate rig, a computer with a radio, along with an app on a smartphone that talks to the pump.
“The way things are set up right now, you’ve got your pump, a CGM, then you’ve got something to bridge the communications between the pump and the CGM. In the US, the only available pumps are the older Medtronic pumps [which can be programmed] so we can set temp basals.”
Tandem Pumps►



Current generation
Tandem t:slim X2
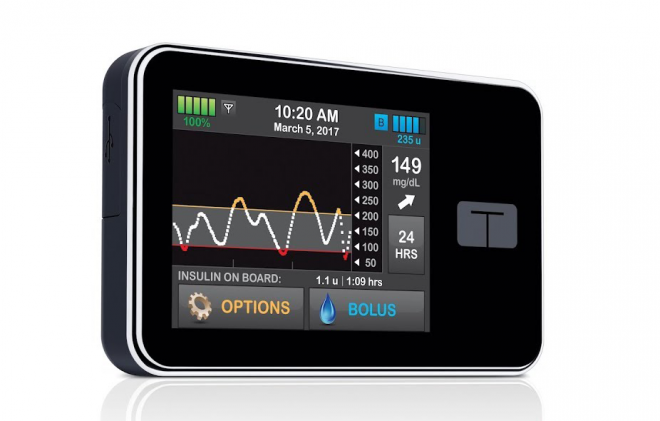
Tandem is pushing out this pump as a solution for people with Type 1 and Type 2 diabetes in multiple countries, advertising it as the smallest tethered insulin pump available on the market and highlighting features such as a touchscreen interface, a seamless software update process, and integration with the Dexcom G6 system. The rechargeable battery lasts up to five days, holds 300 units of insulin and has a maximum bolus setting for up to 25 units at a time. All of these features mean that along with its Basal-IQ algorithm, the t:slim X2 works to reduce the amount of manual work involved in diabetes management along the lines of Medtronic’s hybrid closed-loop pump systems. In December 2019, Control-IQ was cleared for FDA approval and the groundbreaking hybrid closed loop algorithm is expected to launch by the end of January 2020. Available as a free software update for current t:slim X2 users in the US, Control-IQ features adjustments to basal delivery and automatic correction boluses. If you don’t want to use the CGM and Basal-IQ or Control-IQ, PWDs have the option of using the t:slim X2 solo.
Tandem Diabetes Care developed an app to support the pump software. Users can simulate a t:slim X2 or t:flex system with t:simulator, access the t:connect app and update existing pump software right on a smartphone.
Previous Models
Tandem t:flex
Tandem offers three pumps, all of them looking like a smartphone with a sleek look. This brand includes bright color touch screens and compact, thin dimensions, along with software that makes them the smallest available smart pumps. All three pumps are waterproof for a shorter duration than some other pumps: up to three feet in depth for 30 minutes.
Tandem is currently in the process of phasing out their t:flex model, which is the largest-capacity insulin pump on the market. With a cartridge reservoir that holds up to 480 units (the two t:slim models hold 300), it’s designed for people who require more than 100 units of insulin a day. It can calculate blouses up to 60 units at a time and missed bolus reminders can be customized by the day of the week. The t:flex does not offer BGM or CGM interaction, but the t:slim G4 and the t:slim X2 does. Some users report difficulty in hearing the alarms at night, and the aluminum casing around the pump, while adding to its sleek look, can set off metal detectors.
Tandem t:slim G4
The t:slim G4, also sporting shatter-resistant glass, is slightly less bulky than the t:flex. They also feature carb-counting options and integration with T-Connect Diabetes Management Application, Tandem’s web-based tracking software. The cartridge reservoir holds up to 300 units of insulin. This pump comes equipped and paired with the popular Dexcom G4 Platinum CGM. The system is not integrated with Dexcom’s G5 or G6 systems. The
As of publication, Tandem is driving all traffic for the t:slims and the t:flex to the X2 site, although documentation for the G4 remains available.
Roche Pumps►


Accu-Chek Spirit Combo
The Spirit Combo looks like a small grey pager with a simple LCD screen, and the meter display has a color screen. The pump holds 315 unit of insulin and uses a Bluetooth connection to communicate with the Aviva Combo meter, allowing you to control your pump activity remotely. The meter device can deliver insulin from as far as 6.5 feet away. The full-color, on-screen blood sugar and bolus data are easy to read on the Aviva Combo, making it simple and convenient to track your information. The unit holds daily data for up to 90 days. If you are happy with the meter you’re already using and don’t want to use the remote option, the Spirit Combo can also be used independently from the Aviva Combo.
Some of the concerns with using the Spirit and Spirit Combo pump are that neither of the two are waterproof or water resistant or fully integrated with a CGM. While the Spirit Combo has higher insulin capacity than the Animas Ping, the other meter/remote pump combination option on the market, the Ping has a greater range of insulin administration at 10 feet (see below). That might not seem like a great difference, but for parents who are administering insulin to children, that extra three and a half feet could be helpful.
Accu-Chek Spirit
The Spirit is a silver stand-alone pump with a reversible, backlit display that’s relatively easy to read. The design is bare-bones—this pump essentially looks like a small pager. While it lacks some of the features of Accu-Chek’s Spirit Combo Pump, the Spirit holds 315 units of insulin and enough memory to store 30 days of daily readings.
Accu-Chek Insight
The Insight, currently available only in the United Kingdom, builds on the Spirit Combo’s framework and adds a more trendy, sleek design. The Insight is a remote-operated pump featuring a color display and touchscreen technology. It offers a minimum basal rate of 0.02 units/hour, a feature that appeals to families with young children with diabetes.
It holds 200 units of insulin (1.6 ml), and, like the Spirit and Spirit combo, is not CGM integrated or waterproof. Pre-filled 1.6 ml- (160-unit-) cartridges of NovoRapid, called PumpCarts, are available for use with the Insight, which can be convenient for users who want to simplify their infusion set changes. The handset battery, which can be a single lithium or alkaline, only lasts three days.
Accu-Chek Solo
The Solo is a tubeless patch pump currently in development for use in the U.S. It received approval in Europe in July 2018, and Healthline reported that since Roche’s departure from the American insulin pump market in 2017, the Solo product could bring the company back to the United States. Taking a page from the Omnipod, the pump expands on the flexibility of a tubeless pump by offering removability with its adhesive patch, allowing for unplugging when the situation arises. There aren’t any plans as of 2019 to integrate the pump with a CGM, but integration with the Accu-Chek meter system is a given.
Animas Pumps (discontinued in US + Canada)►
Animas Vibe
*Note: This pump has been discontinued in the United States and Canada.
The Animas Vibe pump provides integrated on-screen information so users can closely monitor blood sugar trends. The full-color display makes it easier to see where your blood glucose levels are heading, as opposed to the more traditional green-and-black screen on Medtronic’s Minimed Revel or Minimed Veo. The buttons make this interface bulkier than other full-color pumps, but that can be a positive element when it comes to a child’s ability to navigate the pump. The pump system is approved for children as young as 2 years old and can help with teaching children to independently monitor blood glucose levels, and the 200-unit reservoir capacity might be a benefit for users who require less insulin.
The Animas Vibe is waterproof and has customizable alarms. There is also a built-in, non-adjustable hypoglycemia safety alert set at 55mg/dL. This model is not compatible with the Dexcom G5, but users who want their CGM information available on a pump instead of a smartphone and don’t mind using an older CGM (the G4 Platinum) might find that this system works for them.
Animas OneTouch Ping
*Note: This pump has been discontinued in the United States and Canada.
The main selling point of the Ping is its ease of use. The pump and the meter remote share information wirelessly, allowing you to give yourself an insulin dose without ever touching your pump. Both the pump itself and the remote can control nearly all functions: bolusing, monitoring stats, setting alerts and warnings. This pump also excels at tracking nutritional data along with the meter, which can store the nutritional values of up to 500 foods through the CalorieKing database.
Animas says the meter remote works from up to 10 feet away. The pump is waterproof up to 12 feet in depth for as long as 24 hours, eliminating the need to disconnect while swimming or bathing. Eight different skin covers for pumps and remotes are available to help you match your Ping to your outfit. One drawback of the Ping, however, is that it doesn’t offer interaction with a CGM, like the Animas Vibe. If constant blood glucose monitoring on your pump is what works for you, you’ll have to invest in a second device.


Johnson & Johnson, the Animas manufacturer, is discontinuing the sale of Animas pumps in the United States and Canada amidst a plan to exit the insulin pump market by September 2019. As of the first quarter of 2019, the brand will still remain available outside North America. Animas users, depending on country of residence, can ask their healthcare professionals about the process for upgrading to a Medtronic 670G from their current pump, which is Animas’s recommended transitioning pump.