FDA Approves CamAPS FX Hybrid Closed-Loop Insulin Delivery App
WRITTEN BY: Daniel Trecroci
2024-05-31
The US Food and Drug Administration (FDA) has approved a hybrid, closed-loop, insulin-delivery Android app. On May 29, 2024, CamDiab, a company that creates state-of-the-art diabetes management technologies, revealed that the CamAPS FX had received approval.
CamAPS FX is approved for people with type 1 diabetes who are two years of age or older, including those who are pregnant.
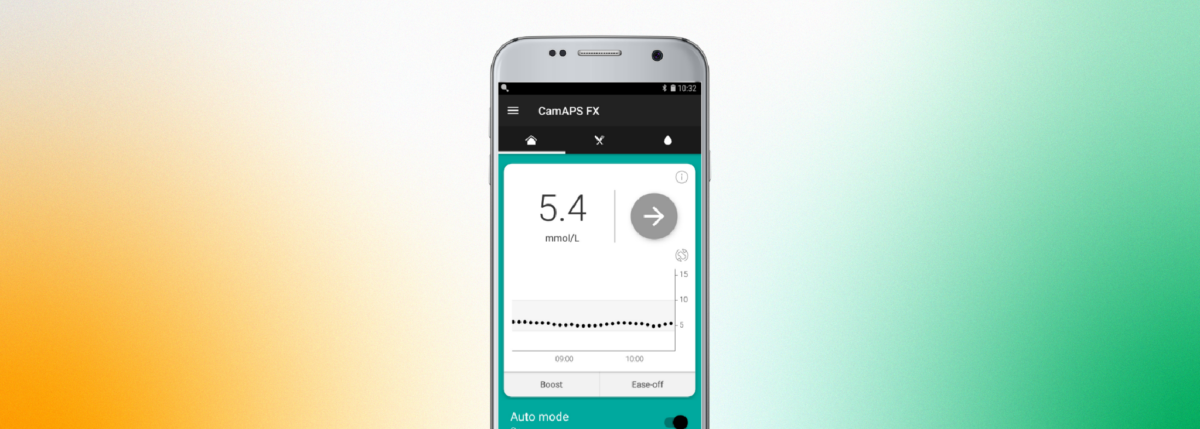
CamAPS FX works in unison with the FreeStyle Libre 3 and the Dexcom G6 to produce a hybrid, closed-loop system that is compatible with the Ypsomed mylife YpsoPump.
The app allows the pump and CGM to speak to one another—creating an “artificial pancreas.”
More than 27,000 people in 15 countries have already used the product, according to CamDiab.
For more information, go to https://camdiab.com.
News coverage by the Beyond Type 1 team is operated independently from any content partnerships.
Beyond Type 1 maintains full editorial control of all content published on our platforms.
WRITTEN BY Daniel Trecroci, POSTED 05/31/24, UPDATED 05/31/24
Dan has written about diabetes for more than 20 years. He was one of Diabetes Health's first recruits, and throughout his 10 + years as Managing Editor he wrote/published thousands of articles and helped establish Diabetes Health as the premiere resource for people with diabetes. He later became the Content Manager for OneTouchGold—Johnson & Johnson/LifeScan’s official digital publication for its metering-technology customers. Under his leadership, OneTouchGold received the Web Marketing Association’s award for “Best Health & Wellness" web site. Dan has also written for the Diabetes Research Institute, dLife, diaTribe, Healthline, CareDx, Pendulum Therapeutics, and Hero Bread.
Read More
Beyond Type 1 provides programs and resources that enhance the lives of those affected by diabetes, ...
Let me tell you why the statement above is a load of crap.
Psychology is the study of how situations, emotions and relationships in our lives interact and...