First Look at Nasal Glucagon BAQSIMI
Written by: Beyond Type 1 Editorial Team
1 minute read
August 11, 2019
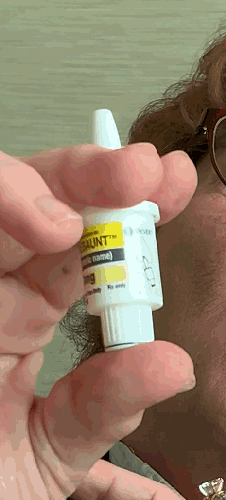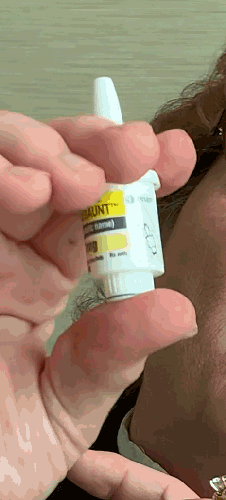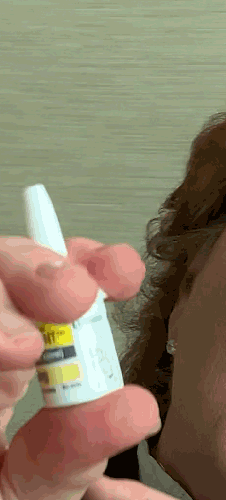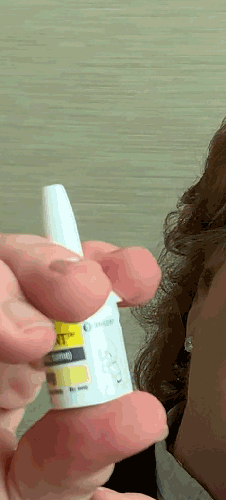 Lilly recently announced that BAQSIMI, the first non-injectable emergency treatment for severe episodes of hypoglycemia, is now available in U.S pharmacies.
Lilly recently announced that BAQSIMI, the first non-injectable emergency treatment for severe episodes of hypoglycemia, is now available in U.S pharmacies.
Today at the AADE 2019 Annual Conference, Beyond Type 1 CEO Thom Scher sat down with former AADE President Deborah Hinnen, APN, of Colorado Springs and U.S. Medical Lead for BAQSIMI, Julie Settles, MSN, APRN for an in-person demonstration of the nasal glucagon device.
Some of the important questions answered in the interview include:
Q) Does BAQSIMI need to be inhaled?
No, BAQSIMI is passively absorbed in the nose, and can be administered to someone who is unconscious.
Q) Will BAQSIMI work on somebody who has a cold?
Yes, BAQSIMI is not affected by a cold, congestion, or concurrent treatment with Afrin nasal spray.
Q) Does BAQSIMI work the same as injected Glucagon?
Yes, BAQSIMI is the same chemical—glucagon—administered through the nasal passages.
Q) Are there different doses of BAQSIMI depending on size?
A single 3mg dose of BAQSIMI works for everyone ages 4 and up.
Watch the entire demonstration and interview below:
For more information on BAQSIMI pricing and availability, CLICK HERE.

Author
Beyond Type 1 Editorial Team
This piece was authored collaboratively by the Beyond Type 1 Editorial Team. Members of that team include Editorial Manager Todd Boudreaux, Program Manager Mariana Gómez, Director of Brand Communications Dana Howe and Editorial Associate Jordan Dakin.
Related Resources
February is American Heart Month. It’s a time to raise awareness about heart disease, which...
Read more

HBO Max’s “The Pitt” has done it again. Known for its real-world depiction of working...
Read more

Super Bowl Sunday is just around the corner, and so are the parties. This yearly...
Read more

