FreeStyle Libre 2 Approved for Use in U.S.
Editor’s Note: In December 2020, Freestyle Libre 2 was approved in Canada for adults and children 4 years and older
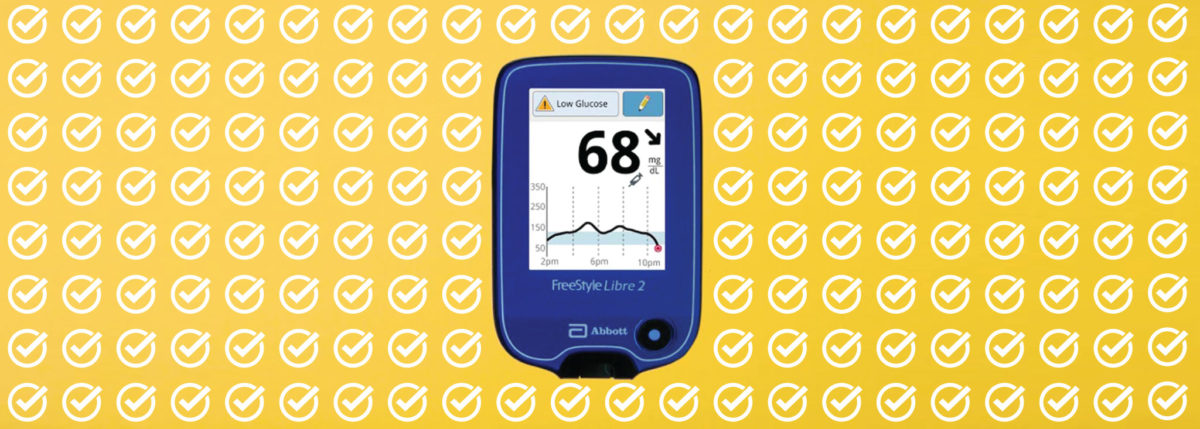
On June 15, 2020, Abbott announced that the U.S. Food and Drug Administration (FDA) has approved the FreeStyle Libre 2 System. The Libre 2 gained its CE Mark clearance in Europe late 2018 and is now approved for adults and children 4 and up in the United States. The FreeStyle Libre 2 is a 14 day continuous glucose monitoring (CGM) system that transmits data every minute and now includes customizable high and low alerts without the need to scan the device. The Abbott announcement says it will be “a third of the cost of other CGMs”.
Also notable with this approval is the FDA’s decision to permit the marketing of the FreeStyle Libre 2 as an integrated continuous glucose monitor (iCGM). This allows the CGM to be part of an interoperable system and work with other medical devices like insulin pumps, automated dosing systems, blood glucose monitors and other devices. This makes the FreeStyle Libre 2 only the second CGM on the market to receive this designation. In February of this year, it was announced that Insulet’s next generation Omnipod 5, powered by Horizon would be compatible with Abbott’s FreeStyle Libre 2.
This approval also marks the first time a FreeStyle Libre CGM has been approved for pediatrics in the US, widening the scope of potential users.
According to Abbott, the Libre 2 adds easy-to-use, customizable alarms for high and low blood glucose levels using Bluetooth. Users can also receive notifications should the sensor and reader fail to link up to ensure that a dropped connection does not go unnoticed. Alerts come in the form of sounds or vibrations, according to preference. Libre 2 users can still scan their sensor as often as desired to see current blood glucose levels (BGs), trends and patterns and a graph of the last eight hours of blood glucose levels.
Though the Libre 2 boasts additional features, it will be available at the same price as the original FSL, which received FDA approval in the U.S. in the fall of 2017 and has been available in Europe since 2014. Abbott’s announcement states that it will be available in the coming weeks through pharmacy and durable medical equipment (DME), likely dependent on your individual insurance plan.
“Managing diabetes is expensive, even for those with insurance coverage,” said Watkin. “From the start, Abbott designed FreeStyle Libre technology with affordability in mind. We set a global price for our sensing technology that’s closer to that of traditional blood glucose fingerstick systems, and significantly less than other CGMs, because we wanted to make sure our life-changing technology was accessible to as many people as possible.”
More breaking news from the 2020 ADA Conference here.