FDA Approves Embecta’s New Insulin-Delivery System for Both Type 1 and Type 2 Diabetes
Written by: Daniel Trecroci
1 minute read
September 3, 2024
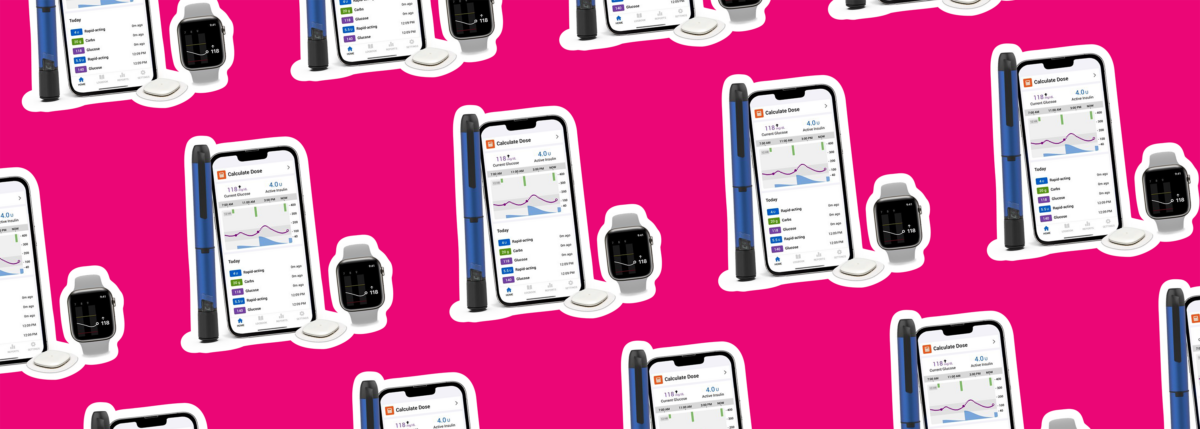
In a significant development for diabetes care, Embecta Corp. has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its groundbreaking disposable insulin-delivery system, designed for adults with both type 1 and type 2 diabetes.
This innovative system features a tubeless patch pump with a 300-unit insulin reservoir, making it particularly suitable for people with type 2 diabetes who require higher daily doses of insulin.
The new system addresses a major gap in diabetes management tools. Although nine out of 10 people with diabetes have type 2 diabetes, most existing automated-insulin-delivery solutions are primarily designed for type 1 diabetes.
Embecta’s new patch pump meets this unmet need with a larger insulin capacity, offering a more convenient option for those moving from multiple daily injections to pump therapy.
A recent study showed that this 300-unit reservoir could meet the insulin needs of 64% of adults with type 2 diabetes for a three-day wear period, compared to just 38% with a 200-unit reservoir.
The fully disposable patch pump provides adjustable basal and bolus insulin delivery for up to three days, offering flexibility and ease of use. The accompanying locked-down controller with Bluetooth® wireless technology and a color touchscreen further enhances the user experience, simplifying diabetes management.
Embecta plans to develop a closed-loop system that includes an insulin-dosing algorithm, aiming for future FDA submissions to expand its product offerings even further.
For more details, visit embecta.com.
Related Resources

Already compatible with Dexcom’s G6 and G7 continuous glucose monitors (CGMs), the Omnipod 5 Automated...
Read more
The younger a person is diagnosed with type 2 diabetes, especially those with obesity, the...
Read more

The Oura Ring, which tracks things like sleep, heart rate, and activity, is joining forces...
Read more