Beta Cell Replacement Therapy: A Pathway to a Cure for Type 1 Diabetes?
Coverage of the American Diabetes Association (ADA) Scientific Sessions is brought to you by the ADA x BT1 Collab.
Speakers for this session, “Emerging Approaches to Beta Cell Replacement Therapy for Type 1 Diabetes,” included: Matthias Hebrok, Ph.D. (University of California, San Francisco), Andrew Pepper, Ph.D. (University of Alberta), Alice Tomei, Ph.D. (Diabetes Research Institute, University of Miami), and Xunrong Luo, MD, Ph.D. (Duke University Medical Center).
In this coverage, we focus on the research presented by Andrew Pepper, Ph.D.
Beta cell replacement therapies beg the question: Are we closer to a cure for type 1 diabetes?
Research is promising.
Andrew Pepper, Ph.D. is studying the current routes to glucose normalization (i.e. blood sugar regulating to a healthy level without administering injections or infused insulin) and the possible advantages and current limitations of beta cell replacement therapy.
Why beta cell replacement therapy?
Though much progress has been made with the artificial pancreas (also known as closed loop or automated insulin delivery systems), we are not where we need to be for it to help people with type 1 diabetes in all ways they need to be helped, Pepper explained. An alternative is beta cell replacement therapy.
“Beta cell replacement therapy has a profound impact on glucose control,” Pepper said, referring to the image below. This data shows results nine months post-transplant (of beta cells).
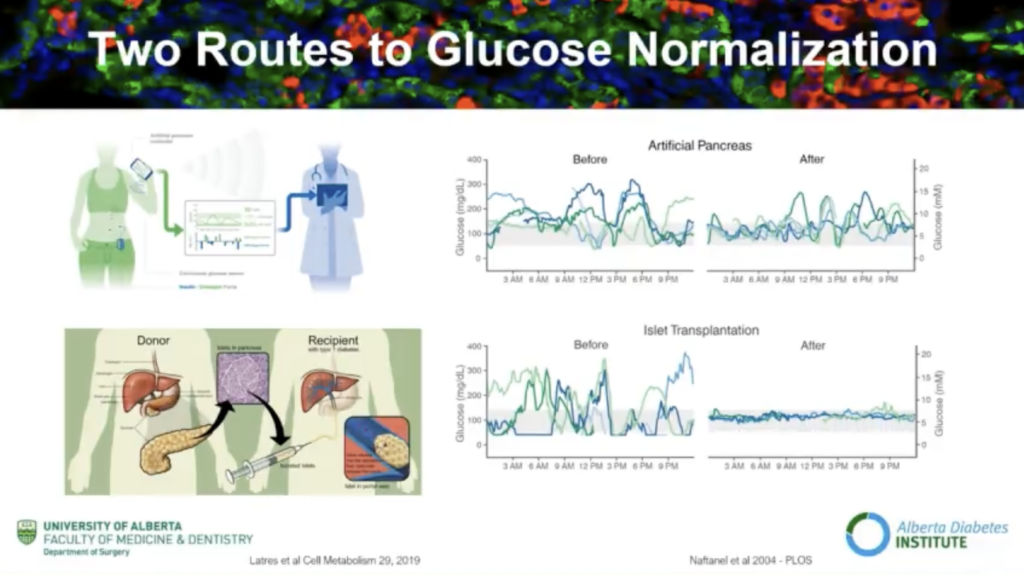
Long-term functioning is achievable with beta cell replacement therapy, Pepper encouraged. Though insulin independence wains over time, you can achieve long-term glucose control with beta cell replacement therapy.
Pepper explained that beta cell replacement therapy is limited to a small subset of patients. Only 5 to 10 percent of individuals living with type 1 diabetes (T1D) fulfill current strict inclusion criteria because of the risks associated with currently needed immunosuppression.
“When we look at the limitations, we are so very close, but so very far away,” Pepper said. “We know that the majority of islets are destroyed hours to days post-transplant. As so, we require multiple donors per recipient to achieve glucose control and insulin independence. We also require life-long anti-rejection drugs.”
The hurdles of beta cell replacement therapy
“Despite the profound research that has been done, the field is still limited due to a limited source of materials (transplantable beta cells) as well as the lifelong need for immunosuppression,” Pepper explained.
Pepper highlighted a few key research areas:
- One of the current advancements is looking at unlimited cell sources. Xenotransplantation has found success in pigs. Heart and kidney xenotransplants have been done successfully, Pepper shared.
- Stem cell therapy is also promising. Pepper noted ViaCyte’s exciting research. Today, they are known for having “the first and only islet cell replacement therapies derived from stem cells in clinical trials for diabetes.”
- Vertex’s work, transplanting SC-beta cells into the liver, has also profoundly impacted the first few patients treated this way. Recently, an individual came off of insulin using this approach.
However, all of these approaches have one problem in common: the need for lifelong systemic immunosuppression. And while type 1 diabetes cure advancements of any kind are worth celebrating, immunosuppressant drugs still pose the threat of the following significant side effects, which should be avoided:
- Loss of appetite
- Nausea and vomiting
- Increased hair growth
- Hand trembling
- Increased risk of infection
- Increased fatigue, weakness, or tiredness
- Fevers and chills
- Frequent urination or a burning sensation when urinating
- A recurring cold or cough with no reprieve
Despite the burden of these side effects, these treatments are still a step forward from history.
The history of beta cell replacement therapy: learning from past studies
“It’s important to know where the field is going, but also have perspective of where we’ve been and what we’ve learned along the way,” Pepper said. “The advent of encapsulation in the ‘70s led to a meteoric rise in encapsulation techniques.”
The goal of many diabetes-related encapsulation studies involves encapsulated islets, which would be able to do the same job as healthy islets—detecting blood glucose level changes and producing insulin—if successful.
Another potential treatment method in this field involves intravascular devices, like the idea of a bionic pancreas. Pepper explained that one of the first intravascular devices used 40 years ago demonstrated an acute restoration of glucose control, but there were significant complications.
“New materials (treatments) may circumvent some of the limitations of their predecessors,” Pepper encouraged.
Microencapsulation studies from 30 years ago that involved rodents led to even more critical studies today. This study showed restoration of glucose control within 15 weeks with no immunosuppression.
This research was a stepping stone for another pivotal study that involved people with type 1 and type 2 diabetes in the early ‘90s, which demonstrated the protection of insulin-producing islets in all forms. However, there was marked degranulation in people with type 1 diabetes.
Though they did not conclude with a cure, these studies are important to recount as they paved the way for future clinical trials in beta cell replacement therapy, and today there are many. Here are some that were featured in the presentation:

Will beta cell replacement therapy lead to a cure?
Often, when new findings arise, so do new concerns.
“When we look at the hurdles, there is potential to overcome limitations with alternative cell sources,” Pepper said. “However, we are still plagued by the biomaterial optimization and chronic fibrosis we see with some of these encapsulation technologies.”
Reducing the foreign body response is critical to the success of encapsulation technologies mentioned throughout this presentation. Certain drugs could help reduce the immune response, but they have not to this day. Delivering oxygen to encapsulated islets also remains a challenge.
Pepper summarized that next-generation techniques show promise in improving encapsulation methods, but research must mitigate the many remaining challenges for them to be effective. Open encapsulation techniques may offer a pathway to insulin independence for people with type 1 diabetes. As new research emerges, we are sure to see even more effective treatments form.
Learn more about Pepper’s contributions to type 1 diabetes cure research and his findings here.





