Research News
2020-03-24
This resource on Type 1 Diabetes Research was created in partnership with JDRF, an active partner of Beyond Type 1 at the time of publication, through the JDRF – Beyond Type 1 Alliance.
Vertex VX-880 Clinical Results Lead To Insulin-Independence
Here's what you need to know about Vertex's recent results from their stem-cell therapy for type 1 diabetes.MORE

T1D Clinical Trial Search Engine Now Available In Spanish
Beyond Type 1 collaborates with Antidote Technologies and JDRF to launch the first-ever Spanish-language clinical trial search engine for type 1 diabetes.MORE

Mounjaro Shows Multiple Benefits for Type 2 Diabetes Management
New, FDA-approved Mounjaro shows promising results for improving several aspects of type 2 diabetes management. MORE

Diabetes Game-Changer: A Once-Weekly Insulin
The development of a once-weekly insulin could simplify diabetes management for those with type 1 and type 2.MORE

Stem Cell Therapy for Type 1 Diabetes: Patient Achieved Insulin Independence on Day 270
A patient in the Vertex Pharmaceutical’s clinical trial using stem-cell therapy to treat type 1 diabetes has achieved insulin independence on Day 270 of the trial. MORE

Dedicated to Helping People With T1D: Dr. Gary Meininger on His Life and Career with Diabetes
We spoke with Dr. Meininger on his personal connection to T1D, his role at Vertex, and important work being done in clinical trials.MORE

Stem-Cell Therapy for Type 1 Diabetes Reduced Patient’s Insulin Needs by 91%
The early results of a clinical trial using stem-cell therapy to treat type 1 diabetes reduced the patient’s insulin needs by 91%.MORE

FDA Issues CRL for Teplizumab, Investigational Candidate to Delay the Onset of T1D
The FDA issues a complete response letter to Provention Bio as part of an update for Teplizumab to delay the onset of type 1 diabetes.MORE

FDA Advisory Committee Votes 10-7 To Recommend Approval of Teplizumab For Delay Of Type 1 Diabetes
Teplizumab is an investigational candidate proven to delay the onset of type 1 diabetes. The FDA advisory committee reviews its efficacy.MORE

The PROTECT Study
The PROTECT Study aims to understand how the investigational medicine, teplizumab, works in children and young adults who have recently been diagnosed with T1D, as well as assessing if there are any s...MORE

FDA Approves New Glucagon Option from Zealand Pharma
Available in June 2021, the FDA approved Zealand Pharma’s Zeagalogue, a new form of glucagon for people with insulin-dependent diabetes.MORE

JDRF Announces New Center of Excellence to Advance T1D Research
On February 23, 2021, JDRF announced the launch of the JDRF Center of Excellence in collaboration with the Harvard Stem Cell Institute in New England.MORE

Congress Renews Funding for Special Diabetes Program
On Dec 22, 2020, Congress renewed the Special Diabetes Program, providing $150 million annually in type 1 diabetes research funding until 2023MORE

JDRF Launches Program to Advance Universal Screening for Type 1 Diabetes
T1Detect is a new screening initiative designed to make early detection of type 1 diabetes easier and more accessible to a broad population. MORE

From Diagnosis Through Complications: Disparities in Diabetes Care
Recent research is confirming what many have experienced - that beginning at diagnosis, ethnic minorities in the US face more challenges navigating diabetes and care than their white counterparts. MORE

JDRF Announces Updated Strategy, Renewed Focus in Light of COVID-19
Amidst COVID-19, JDRF prioritizing work in their portfolio that they see as having the highest likelihood for cures and life changing advances for diabetes.MORE
Omnipod Closed Loop Technology to be Compatible with Both Abbott and Dexcom CGMs
Abbott and Dexcom announced formal partnerships with Insulet to be compatible as CGMs on the Omnipod Horizon Automated Insulin Delivery System. MORE

New Evidence Shows Vital Importance of Screening For T1D in Children
By screening for type 1 diabetes in children, Bavaria was able to decrease rates of diabetic ketoacidosis at diagnosis.MORE

vTv Therapeutics Announces Positive Study Results of Potential First-in-Class Oral Adjunctive Therapy for T1D
On February 10, vTv Therapeutics announced positive study results from the second part of the Phase 2 Simplici-T1 Study of TTP399.MORE

JDRF CEO to Health Insurers: Cover New Technology
JDRF President & CEO Aaron Kowalski, Ph.D calls on insurers to cover FDA approved type 1 diabetes technologyMORE
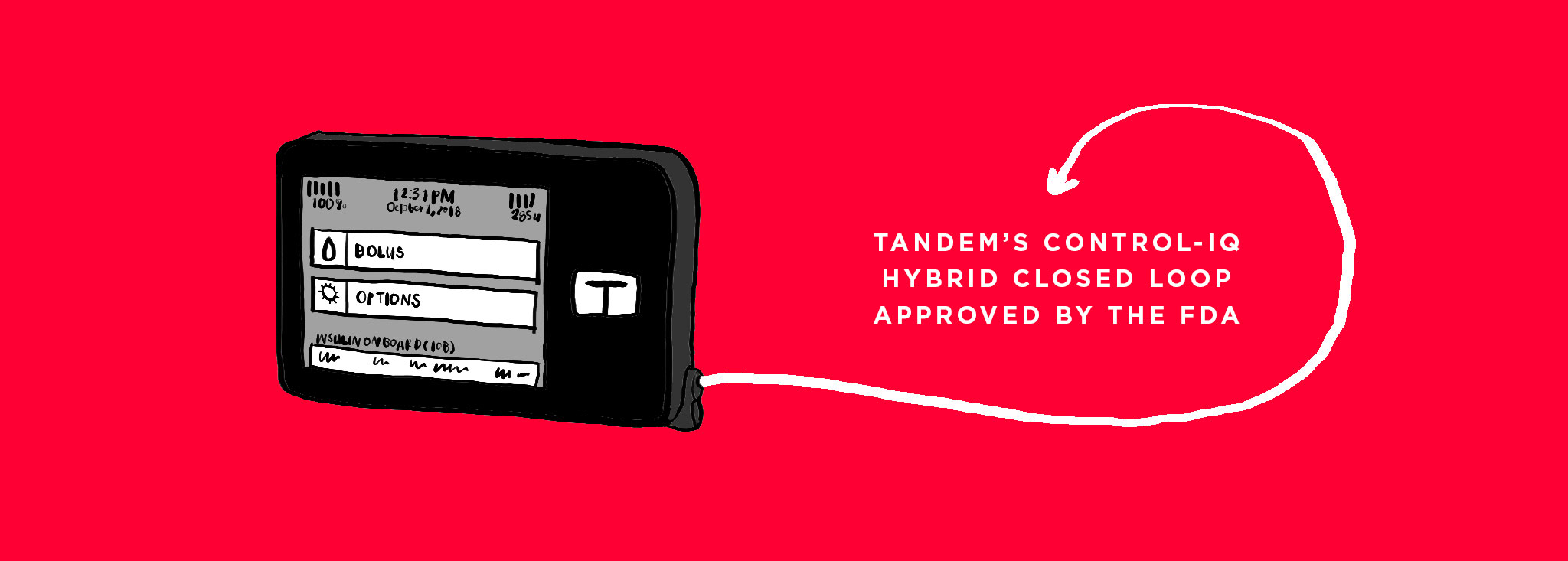
Tandem’s Control-IQ Hybrid Closed Loop Approved by the FDA
The FDA announced the approval of the Control-IQ Hybrid Closed Loop System from Tandem Diabetes Care. The system sees the t:slim X2 insulin pump and Dexcom G6 continuous glucose monitor working togeth...MORE
Implanted PEC-01 Cells Capable of Producing Insulin According to New Data Presented from ViaCyte
ViaCyte presented preliminary data from their PEC-Direct trial, showing that PEC-01 cells are capable of producing insulin in patients with type 1 diabetes.MORE

JDRF Launches Center of Excellence to Accelerate Cure for Type 1 Diabetes
On September 4, JDRF announced the launch of its Northern California Center of Excellence, dedicated to focused efforts on research and breakthroughs to cure type 1 diabetes.MORE

Preventing Type 1 Diabetes: Results From TrialNet’s Landmark Teplizumab Trial
The immunotherapy drug teplizumab was shown to delay type 1 diabetes diagnosis a median of 2 years in children and adults at high risk.MORE

Nonprofits Announce Groundbreaking Research Initiative
The Parker Institute, JDRF and The Leona M. and Harry B. Helmsley Charitable Trust announced a research initiative to study immunotherapy-induced diabetes. MORE

FDA Approves New Treatment for Diabetic Retinopathy
Regeneron Pharmaceuticals announced that the FDA has approved EYLEA (aflibercept) to treat all stages of diabetic retinopathy.MORE
To learn more about all the great type 1 diabetes (T1D) research being funded by JDRF, visit their research and impact page here.