The Price of Uncertainty: Unveiling the Impact of Unexpected Type 1 Diabetes Diagnoses
Written by: Beyond Type 1 Editorial Team
4 minute read
June 5, 2024
Receiving a type 1 diabetes (T1D) diagnosis can be life-altering, bringing with it many visible and invisible costs.
Findings from a new survey commissioned by Sanofi, called “The Cost of Not Knowing,” have shed light on the profound impact of receiving a T1D diagnosis without prior warning. The findings, which were announced on June 5, 2024, highlight the social, emotional, and financial costs associated with unexpected diagnoses while also outlining a pathway to empowerment via early screening and education. Autoantibody screening is a blood test that looks for diabetes-related autoantibodies to see if someone is at risk for developing T1D.
What if There Was More Time to Prepare for a T1D Diagnosis?
“The Cost of Not Knowing” explores the impact of a T1D diagnosis on caregivers of children under the age of 18 who also have the condition, as well as adults with T1D over the age of 18.
The aim of the survey research was to examine in more detail the apparent and invisible consequences of an unexpected T1D diagnosis, and to determine if early detection using autoantibody screening could have mitigated these effects.
By taking the step to have ourselves, our children, or our family members screened for T1D, we may give ourselves more time to prepare and plan for the future. It’s important to talk to your doctor about screening.
Key Survey Findings from The Cost of Not Knowing:
The Heavy Weight of Hindsight: Caregivers and Adults Wish They Had Known Sooner
“The Cost of Not Knowing” reveals a poignant truth: most people with T1D carry the heavy burden of regret, and wish they had known about the risks sooner.
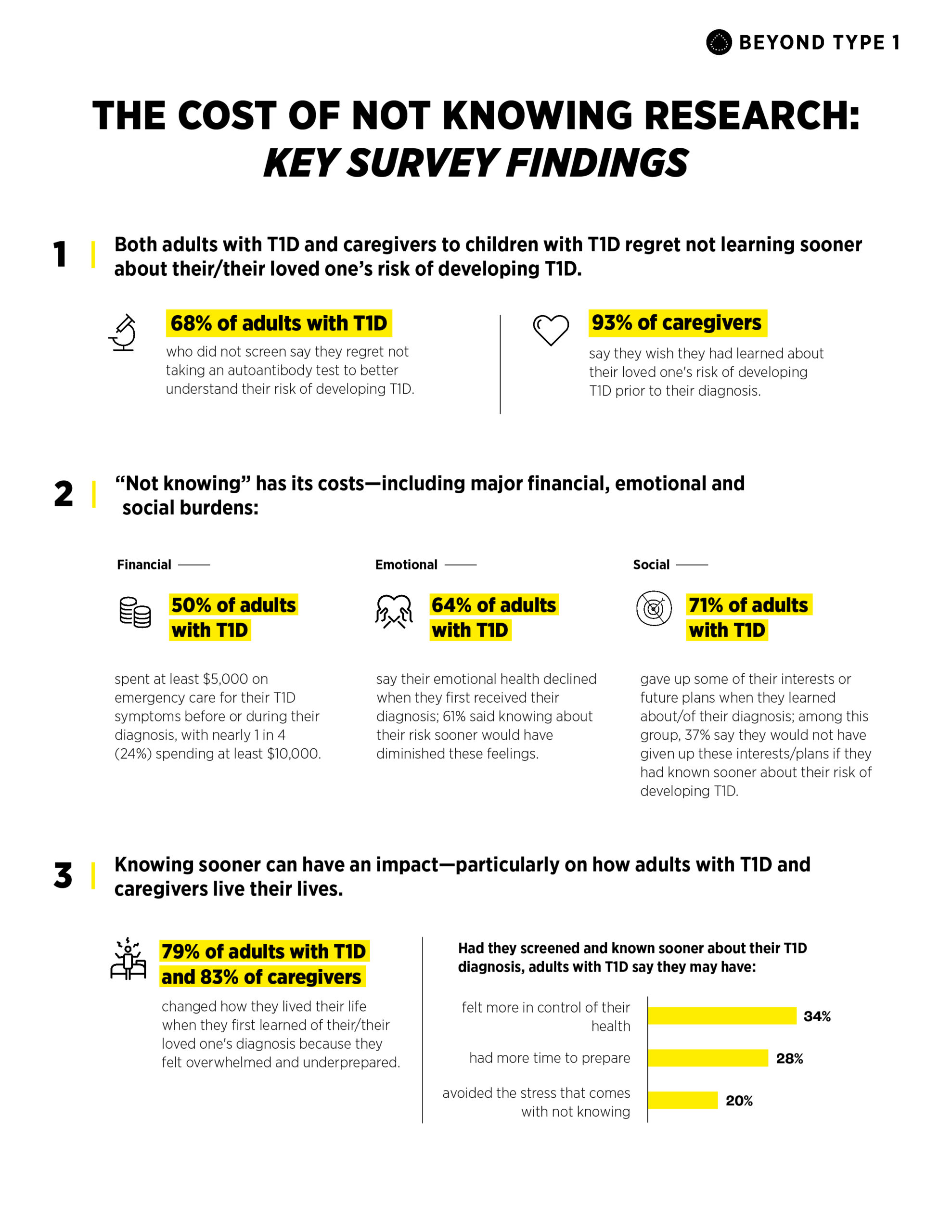
- 68% of adults with T1D who were not screened say they regret not having an autoantibody test to determine their risk of developing the disease.
- 93% of caregivers say they wish they had learned about their loved one’s risk of developing T1D prior to their diagnosis.
The Far-Reaching Impact: Emotional, Social, and Financial Costs of Unexpected Diagnoses
The toll of an unexpected T1D diagnosis extends far beyond the physical, affecting emotional well-being, social lives, and financial stability.
- Emotional: 64% of adults with T1D said their emotional health declined upon diagnosis. 61% who experienced feelings such as sadness, fear, anger, or uncertainty believe knowing their risk sooner would have diminished these feelings.
- Social: 87% of caregivers and 71% of adults with T1D gave up some of their interests or future plans when they learned of their diagnosis.
- Financial: 50% of adults with T1D spent at least $5,000 on emergency care for their symptoms before or during diagnosis, with nearly 1 in 4 (24%) spending at least $10,000.
Empowering Lives: The Transformative Potential of Early Screening
Imagine a world where individuals and families facing T1D had the power of an early diagnosis on their side. This survey research sheds light on the profound difference early screening could make.
- 79% of adults with T1D and 83% of caregivers changed how they lived their life when they first learned of their/their loved one’s T1D diagnosis because they felt overwhelmed and underprepared.
- Had they screened and known sooner about their T1D diagnosis, 85% of adults with T1D think their life may have been different:
- 34% say they might have felt more in control of their health
- 28% say they may have had more time to prepare
- 20% say they may have avoided the stress that comes with not knowing.
These results reveal the striking potential benefits of early screening. Another startling discovery is that 64% of adults with T1D who underwent autoantibody screening reported their emotional health improved when they received their diagnosis. When these adults were diagnosed:
- Only 26% of them felt fearful.
- Only 10% felt uncertain about the future.
In contrast, among those who did not have the autoantibody test for T1D:
- 40% felt fearful
- 28% felt uncertain about the future.
The Invisible Barriers: Access Barriers Prevent Making Autoantibody Screening Common Practice
Despite these clear impacts, these findings also expose both a lack of awareness and perceived barriers preventing access to early T1D screening.
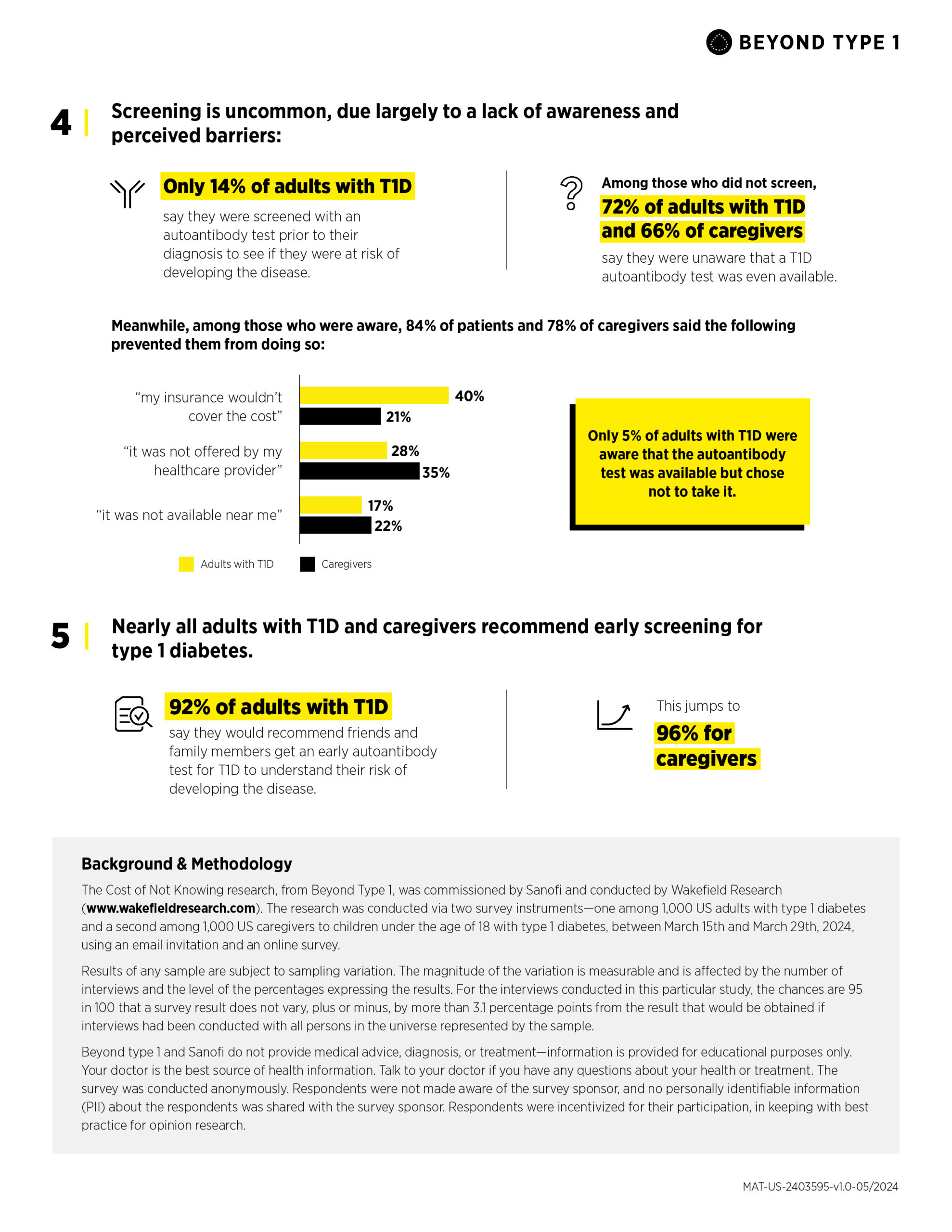
- Only 14% of adults with T1D say they were screened with an autoantibody test prior to diagnosis to see if they were at risk of developing the disease.
- Furthermore, among those who did not screen—or whose loved one did not screen—72% of adults with T1D—and 66% of caregivers—report that they were unaware that a T1D autoantibody test was even available.
- Among those who were aware, 84% of adults with T1D and 78% of caregivers say that barriers prevented them/their loved one from screening, including insurance (40% and 21%) and screening not being offered by their healthcare provider (28% & 35%).
It’s clear that we must work to remove barriers to screening access and prioritize widespread education. No one should have to face a life-altering diagnosis unprepared.
A Resounding Call for Change: Nearly All Adults with T1D and Caregivers Recommend Early Screening
These results point to an urgent need for action, with nearly all adults with T1D (92%) and caregivers (96%) recommending early autoantibody testing to friends and family to better understand their risk of developing T1D.
Anyone can develop T1D at any time, with about 90% of those diagnosed having no family history. By advocating for change, championing early screening, and connecting those at risk with resources like Beyond Type 1, we can transform the T1D journey from one of fear and isolation to one of empowerment and support.
No one should have to experience the price of uncertainty. Through early autoantibody screening, we can help ensure that no one has to face this life-altering diagnosis unprepared.
Take action today – talk to your doctor about autoantibody screening and become an advocate.
Learn more about “The Cost of Not Knowing” below.
Background & Methodology
The Cost of Not Knowing research, from Beyond Type 1, was commissioned by Sanofi and conducted by Wakefield Research (www.wakefieldresearch.com).
The research was conducted via two survey instruments—one among 1,000 US adults with type 1 diabetes and a second among 1,000 US caregivers to children under the age of 18 with type 1 diabetes, between March 15th and March 29th, 2024, using an email invitation and an online survey.

Author
Beyond Type 1 Editorial Team
Beyond Type 1 is the largest diabetes org online, funding advocacy, education and cure research. Find industry news, inspirational stories and practical help. Join the 1M+ strong community and discover what it means to #LiveBeyond a diabetes diagnosis.
Related Resources

On November 20, 2024, Medtronic received FDA clearance for its latest InPen app. This advancement...
Read more

Eli Lilly and Company is helping patients and caregivers understand important changes to Medicare Part...
Read more

Already compatible with Dexcom’s G6 and G7 continuous glucose monitors (CGMs), the Omnipod 5 Automated...
Read more

